空氣凈化工程4大應用領域的特點
- 發布人:
- 發布日期:2017-04-13 17:16:32
空氣凈化工程應用范圍較廣的四大領域主要是指:電子工廠、制藥廠、手術室、生物安全實驗室,不同領域凈化控制系統要求各不相同,同時這些領域控制標準可廣泛應用于其他行業,如電子工廠凈化工程的控制系統可用于PCB生產車間、電器、注塑車間等。四大領域空氣凈化工程的控制特點有以下不同:
Air purification engineering application scope of the four major areas are: electronic factory, pharmaceutical factory, operation room, biological safety laboratory, different areas of purification control system requirements vary, and these areas can be widely used in other industries, such as electronic engineering control system can be used for PCB production workshop, electrical, injection molding workshop, etc.. The control characteristics of air purification project in the four major areas are as follows:
電子工廠潔凈度直接影響到電子產品的質量,采用一次送風、二次送風系統,大量使用FFU風機過濾機組層層凈化空氣,達到更衣室8級凈化、走廊走道7級、生產車間6級,局部需要5級或4級之間,在工程完工后需要靜態驗收。
Electronic factory cleanliness directly affects the quality of the electronic products, the use of a wind, the two air supply system, a large number of FFU fan filter units to purify the air, to reach the 8 level of the dressing room, corridor 7, production workshop 6, 5 or 4 local level, after the completion of the project need to static acceptance.
制藥廠凈化控制三個目標分別是潔凈度,氣閘室廣泛使用六級標準,控制潔凈室內無交叉污染;CFU是指潔凈室內活菌個數;工程需要獲得國家GMP認證;完成了這三個目標才能是一項合格的凈化工程項目,工程系統需用值班風機局部5極,由藥檢局動態監控,靜態驗收后使用。
Pharmaceutical factory pollution control three goals are cleanliness, airlock widely used six grade standard, control the clean indoor no cross contamination; CFU is refers to the clean indoor living bacteria number; engineering need access to national GMP certification, completed the three goals to is a qualified chemical net project, engineering system required duty fan local 5 pole, by the drug administration dynamic monitoring, static acceptance.
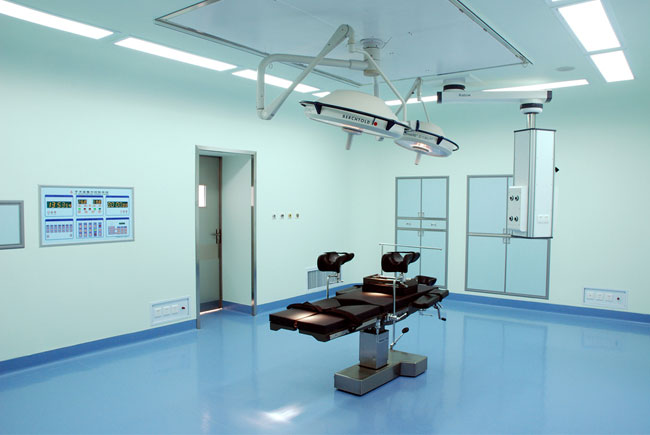
手術室控制較為嚴格,采用MAU+AHU系統,送風天花3m*3m,上千級凈化級別,驗收標準為靜態驗收,動態驗收溫度、濕度、CFU。通常情況下患者在做手術時感染率為25%,根據統計目前手術感染率達10%-15%,許多醫院以日常手術感染率為考核手術室凈化工程的優良指標。
Operation room control is more stringent, the use of MAU+AHU system, send the wind 3m*3m, thousands of purification level, acceptance criteria for static acceptance, dynamic acceptance temperature, humidity, CFU. The infection rate is 25%, according to the statistics of the current surgical infection rate of 10%-15%, many hospitals in the daily operation of the evaluation of the quality of the operation room.
生物安全實驗室凈化工程控制特點我國已制訂相關安全條令,空氣凈化工程 http://www.jnjkjh.com/ 設備選用生物安全隔離服、獨立供氧系統,廢液、廢氣統一凈化通過嚴密性試驗后處理,采用密閉隔離器,負壓二級屏障系統,保障人身安全,并制訂好完善的生物安全實驗室凈化管理條令。
Laboratory biosafety purification engineering control characteristics of our country has formulated the relevant safety regulations, air purification engineering equipment selection of biosecurity quarantine service, independent of oxygen supply system, unified of waste liquid and gas purification through the treatment of the tightness test, the airtight isolator, negative pressure secondary barrier system, security personal safety, and formulate perfect biosafety laboratory decontamination management regulations.
- 上一篇:凈化鋁材配件-無菌物品的管理
- 下一篇:了解潔凈廠房設計施工消毒滅菌的相關知識